LUYOR-3109高強度紫外催化光源促銷
LUYOR-3109紫外光源采用了9顆365nm大功率led,安裝有二次光學透鏡,輸出紫外線強度高,...
2024-08-08作者:生命科學事業部時間:2019-10-07 15:50:49瀏覽12329 次
紅色熒光蛋白(RFP)是從太平洋與海葵相關的 (Discosomasp)中分離出來的一種能在紫外線的照射下可發射紅色熒光的蛋白。在細胞中熒光轉換效率高.是目前發現的具有最長激發波長與發射波長的自然發光熒光蛋白,RFP基因編碼的蛋白質由225個氨基酸組成,相對分子質量(Mr)為25.9ku.RFP以被廣泛用于動物、植物和酵母等真核細胞內基因表達,其基因在原核細胞中的應用也越來越受關注
紅色熒光蛋白(RFP)是從太平洋與海葵相關的 (Discosomasp)中分離出來的一種能在紫外線的照射下可發射紅色熒光的蛋白。在細胞中熒光轉換效率高.是目前發現的具有最長激發波長與發射波長的自然發光熒光蛋白,RFP基因編碼的蛋白質由225個氨基酸組成,相對分子質量(Mr)為25.9ku.RFP以被廣泛用于動物、植物和酵母等真核細胞內基因表達,其基因在原核細胞中的應用也越來越受關注。紅色熒光蛋白RFP是的一種生物發光蛋白,由DsRed和增強型黃色熒光蛋白(CFP),所以RFP作為一種新型可視性標簽在真核基因表達、目的蛋白的細胞及亞細胞定位等研究中得到廣泛應用 。然而,RFP的缺點是需要形成結構穩定的同源四聚體、且需要紫外光等特殊光源的激發才能發射出紅色熒光,尤其是RFP容易形成難溶性多聚體 。DsRed2是源于野生型DsRed的一種人工突變基因,該突變基因的表達產物RFP2,其蛋白結構與綠色熒光蛋白(GFP)相似;與DSRED基因編碼的RFP 蛋白(氨基酸殘基數為248)相比,RFP2蛋白氨基酸序列的C?末端少23 個氨基酸,其發光基團的成熟速度更快,形成的難溶性多聚合體也有所減少。
紅色熒光蛋白激發光與發射光波長:
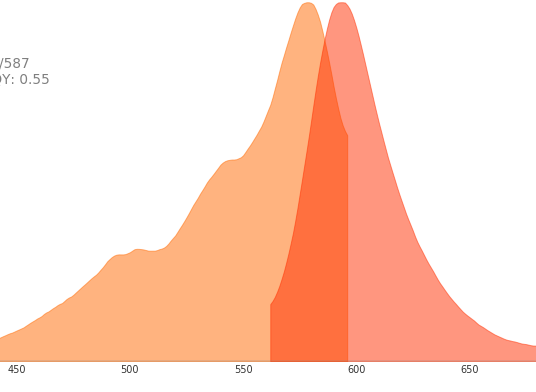
如何選擇合適的RFP(DsRed)標簽抗體?
我們在選擇RFP(DsRed)抗體時,主要根據自己的需要,特別是實驗應用上的需要。另外,一個抗體檢測后的應用類型越多,在使用過程中的選擇余地就越大;而小鼠源的單克隆抗體一般在特異性、穩定性以及效價都要優于多克隆抗體。 另外,抗體的效價也是考察該抗體性價比的重要方面,也即相當于實際應用時的稀釋比率。一個效價高的100μl RFP抗體(假如其WB檢測的稀釋比率是1:5000,最終使用液相當于500ml),要比效價低的1ml的RFP抗體(假如WB檢測的稀釋比率是 1:200,最終使用液相當于200ml)性價比更高。
如果要觀察Dsred紅色熒光蛋白(red Fluorescent Protein)的表達,美國路陽生產的便攜式熒光蛋白激發光源可以選擇LUYOR-3260GR和LUYOR-3415(X)G系列雙波長熒光蛋白激發光源。紅色熒光蛋白采用綠光激發,佩戴LUV-50A紅色觀察眼鏡觀察,如希望提供更多詳細信息,可直接聯系上海路陽生物技術有限公司的銷售客服。

上圖為LUYOR-3415RG便攜式紅色熒光蛋白激發光源

上圖為LUV-50A紅色熒光蛋白觀察眼鏡

上圖為LUV-590A 紅色熒光蛋白拍照濾鏡
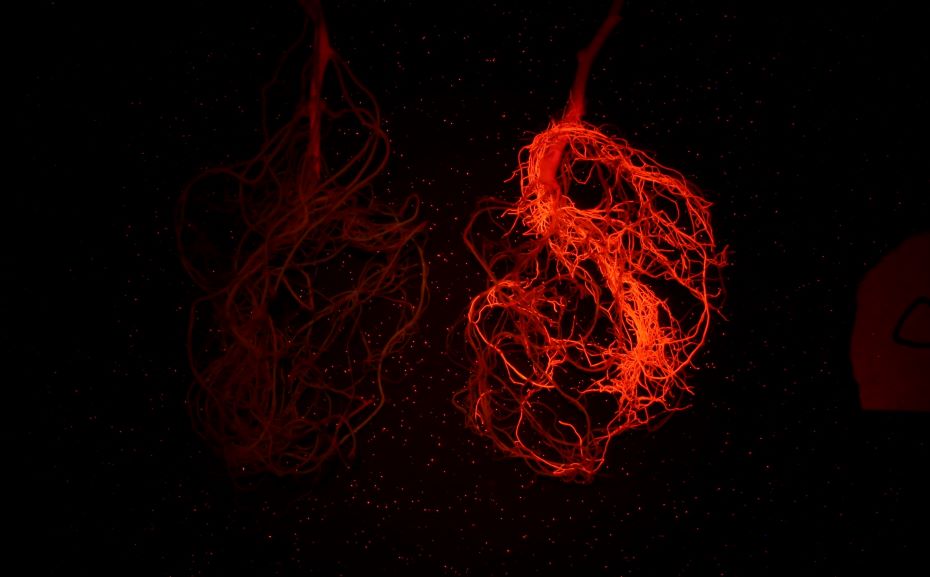
上圖為紅色熒光蛋白在大豆根系上的表達(LUYOR-3415RG照射,LUV-590A濾鏡拍攝)
A major goal of fluorescent protein development has become the construction of a red-emitting derivative that equals or exceeds the advanced properties of enhanced green fluorescent protein. Among the advantages of a suitable red fluorescent protein are the potential compatibility with existing confocal and widefield microscopes (and their filter sets), along with an increased capacity to image entire animals, which are significantly more transparent to red light. Because the construction of red-shifted mutants from the Aequorea victoria jellyfish green fluorescent protein beyond the yellow spectral region has proven largely unsuccessful, investigators have turned their search to the tropical reef corals.
The first coral-derived fluorescent protein to be extensively utilized was derived from Discosoma striata and is commonly referred to as DsRed. Once fully matured, the fluorescence emission spectrum of DsRed features a peak at 583 nanometers whereas the excitation spectrum has a major peak at 558 nanometers and a minor peak around 500 nanometers. Several problems are associated with using DsRed, however. Maturation of DsRed fluorescence occurs slowly and proceeds through a time period when fluorescence emission is in the green region. Termed the green state, this artifact has proven problematic for multiple labeling experiments with other green fluorescent proteins because of the spectral overlap. Furthermore, DsRed is an obligate tetramer and can form large protein aggregates in living cells. Although these features are inconsequential for the use of DsRed as a reporter of gene expression, the usefulness of DsRed as an epitope tag is severely limited. In contrast to the jellyfish fluorescent proteins, which have been successfully used to tag hundreds of proteins, DsRed conjugates have proven much less successful and are often toxic.
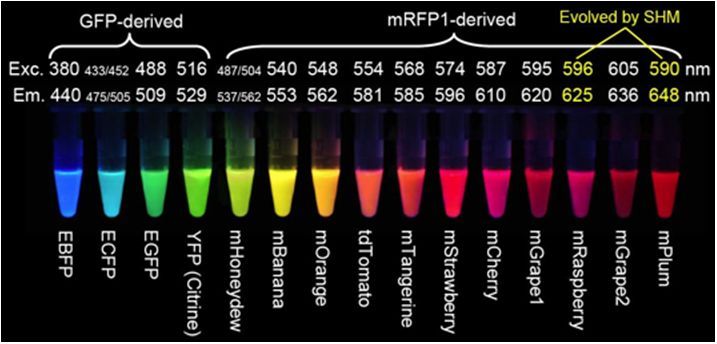
A few of the problems with DsRed fluorescent proteins have been overcome through mutagenesis. The second-generation DsRed, known as DsRed2, contains several mutations at the peptide amino terminus that prevent formation of protein aggregates and reduce toxicity. In addition, the fluorophore maturation time is reduced with these modifications. The DsRed2 protein still forms a tetramer, but it is more compatible with green fluorescent proteins in multiple labeling experiments due to the quicker maturation. Further reductions in maturation time have been realized with the third generation of DsRed mutants, which also display an increased brightness level in terms of peak cellular fluorescence. Red fluorescence emission from DsRed-Express can be observed within an hour after expression, as compared to approximately six hours for DsRed2 and 11 hours for DsRed. A yeast-optimized variant, termed RedStar, has been developed that also has an improved maturation rate and increased brightness. The presence of a green state in DsRed-Express and RedStar is not apparent, rendering these fluorescent proteins the best choice in the orange-red spectral region for multiple labeling experiments. Because these probes remain obligate tetramers, they are not the best choice for labeling proteins.
紅色熒光蛋白的激發波長和發射波長Red Proteins
| Protein | Excitation Wavelength | Emission Wavelength |
|---|---|---|
| TagRFP | 555 | 584 |
| TagRFP-T | 555 | 584 |
| RRvT | 556 | 583 |
| mRuby | 558 | 605 |
| mRuby2 | 559 | 600 |
| mTangerine | 568 | 585 |
| mApple | 568 | 592 |
| mStrawberry | 574 | 596 |
| FusionRed | 580 | 608 |
| mCherry | 587 | 610 |
| mNectarine | 558 | 578 |
| mRuby3 | 558 | 592 |
| mScarlet | 569 | 594 |
| mScarlet-I | 569 | 593 |