LUYOR-3109高強度紫外催化光源促銷
LUYOR-3109紫外光源采用了9顆365nm大功率led,安裝有二次光學透鏡,輸出紫外線強度高,...
2024-08-08作者:激發光源事業部時間:2019-10-11 16:52:17瀏覽13571 次
經常有客戶咨詢BFP(Blue Fluorescent Protein)的激發波長和發射波長,現在將BFP的激發波長和發射波長的光譜圖提供給大家參考。BFP的激發波長在400nm左右,發射波長在450nm左右。
美國路陽生產一款便攜式熒光蛋白激發光源,能夠方便快捷觀察EGFP在動植物體內的表達。我們免費提供樣機供科研院所試用,滿意付款。如有興趣,可添加微信號(luyor01)聯系。

EGFP在煙草上的表達
綠色熒光蛋白在果蠅上的表達
經常有客戶咨詢BFP藍色熒光蛋白(Blue Fluorescent Protein)的激發光波長和發射光波長,現在將BFP藍色熒光蛋白的激發光波長和發射光波長的光譜圖提供給大家參考。
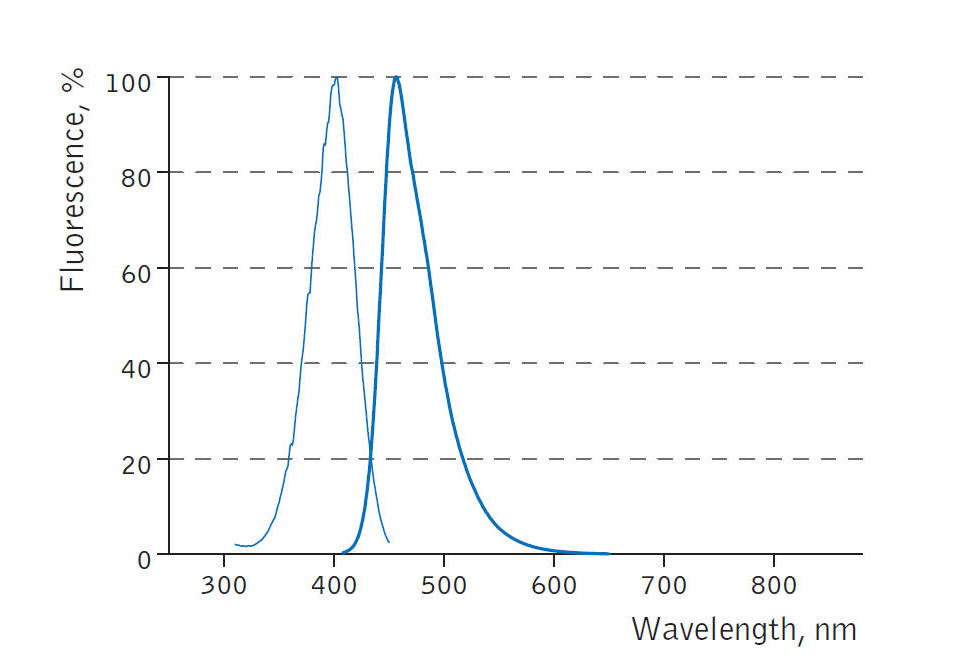
BFP藍色熒光蛋白的激發和發射光譜圖
如果要觀察BFP藍色熒光蛋白(Blue Fluorescent Protein)的表達,美國路陽生產的便攜式熒光蛋白激發光源可以選擇LUYOR-3260VI和LUYOR-3415VX系列雙波長熒光蛋白激發光源。藍色熒光蛋白采用美國路陽紫光激發,佩戴LUV-20A綠色觀察眼鏡觀察,如希望提供更多詳細信息,可直接聯系上海路陽生物技術有限公司的銷售客服。

| 熒光蛋白名稱 | 激發光波長nm | 發射光波長nm |
|---|---|---|
| TagBFP | 402 | 457 |
| mTagBFP2 | 399 | 454 |
| Azurite | 383 | 450 |
| EBFP2 | 383 | 448 |
| mKalama1 | 385 | 456 |
| Sirius | 355 | 424 |
| Sapphire | 399 | 511 |
| T-Sapphire | 399 | 511 |
藍色和青色熒光蛋白變種是將綠色熒光蛋白的66位的酪氨酸殘基突變為組氨酸形成的。這一轉變使藍色發射光更大波長為450nm,而突變為色胺后,熒光的峰值在480nm。這兩個熒光蛋白的熒光都很弱,需要改造來提高折疊效率和熒光亮度。盡管進行了改造,兩者的熒光蛋白亮度僅為增強型綠色熒光蛋白的25-40%。除此之外,藍色和青色熒光蛋白在不常用的光譜區應用起來很有效,所以需要特殊的濾光片和激光光源。
除了以上缺點,在多顏色熒光標記和FRET實驗中這兩種熒光蛋白得到了較多的應用。尤其是增強型青色熒光蛋白,可以被氬離子激光激發脫離峰值(用457nm光譜線),而且比藍色衍生物更能抵抗光漂白作用。跟其他熒光蛋白相比,對可見光藍色區域的熒光蛋白并沒有引起很高的重視,在這一組熒光蛋白中主要研究集中在青色熒光蛋白中。
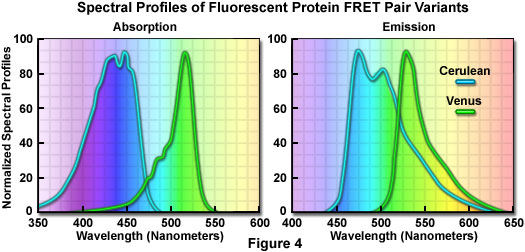
在所有改進過的青色熒光蛋白中,AmCyan1和Cerulean兩個變種最有應用前景。AmCyan1熒光蛋白變種來源于Anemonia majano。跟增強型青色熒光蛋白在哺乳動物表達中相比,已經人源化產生了亮度相對高并可以抵抗光漂白的熒光蛋白。與其他的珊瑚蛋白相似,這種熒光探針也易于形成四聚體。Cerulean熒光探針已經通過對增強型青色熒光蛋白定點突變開發出來,產生了一個高消光系數和量子產量提高的變種。Cerulean的亮度至少為增強型青色熒光蛋白的兩倍;在TRET中,也證實與黃色熒光蛋白一起使用時,大大提高了信噪比。
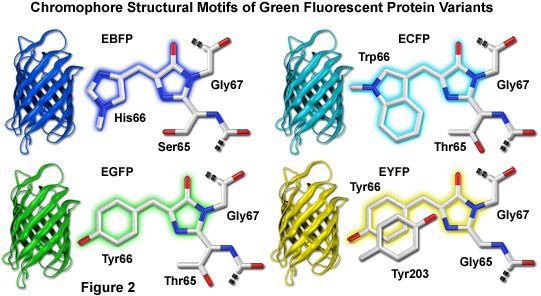
Fluorescent proteins emitting in the blue region of the visible light spectrum (approximately 440 to 470 nanometers) were first obtained from site-directed mutagenesis efforts targeted at the tyrosine amino acid residue at position 66 in the GFP chromophore (see Figure 2). Conversion of this residue to histidine (Y66H) produces a blue fluorescent protein (BFP) that exhibits a broad absorption band in the ultraviolet centered close to 380 nanometers and an emission maximum at 448 nanometers. The original protein exhibited only about 15 to 20 percent of the parent GFP brightness value due to a low quantum yield and required additional secondary mutations to increase its folding efficiency and expression levels. Subsequent investigations and several additional mutations led to an enhanced BFP version that is still only 25 percent as bright as the enhanced green variant and displays limited photostability compared to many other fluorescent proteins.
The primary motivation for developing blue fluorescent proteins in the mid-to-late 1990s was the keen interest in creating matched pairs for fluorescence resonance energy transfer (FRET) experiments and multicolor labeling. Because the spectral characteristics (fluorescence emission profile) of BFP are readily distinguishable from EGFP, this protein combination was one of the first utilized for multicolor imaging. Blue fluorescent protein also has the distinction of being incorporated into the first genetically encoded biosensor along with an enhanced GFP variant to demonstrate FRET through linkage of the two fluorescent proteins via an intervening protease-sensitive spacer. The broad emission peak of BFP overlaps to a significant extent with the excitation spectrum of red-shifted GFP variants to yield a F?rster distance of 4.1, a reasonable value for measuring FRET. Blue fluorescent protein has also been coupled with several GFP derivatives into biosensors designed to monitor transcription factor dimerization, calcium, and apoptosis.
Aside from the limited brightness levels and rapid photobleaching, blue fluorescent proteins also suffer from the fact that they must be excited with ultraviolet light, which is phototoxic to mammalian cells, even in limited doses. Furthermore, working in this spectral region is often hampered by autofluorescence and high absorption levels by cells and tissues, as well as light scattering. Microscopes operating in the ultraviolet also require specialized light sources, optics, and filter combinations that further complicate imaging. For all of the reasons listed above, the quest for more efficient blue fluorescent proteins has only been pursued by a few research groups. Investigations of mutagenesis using non-natural amino acids positioned in and around the chromophore have led to several blue-shifted "artificial" fluorescent protein variants that may find utility in several biological and photophysical applications.
Illustrated in Figure 2 are the chromophore structures for color variants of green fluorescent protein. In all cases, the first step in maturation of the chromophore is a series of torsional adjustments that relocate the carboxyl carbon of the amino acid at position 65 so that it is in close proximity to the amino nitrogen of the glycine residue at position 67 (Gly67) in the polypeptide backbone. Nucleophilic attack by this carbon atom on the amide nitrogen of glycine, followed by dehydration, results in formation of an imidazolin-5-one heterocyclic ring system. Fluorescence occurs when oxidation of the aromatic amino acid (position 66) carbon bond by molecular oxygen extends electron conjugation of the imidazoline ring system to include the aromatic substituent. The cyan, green, and yellow fluorescent protein variant chromophores are discussed in greater detail in the following sections.